AQA GCSE Static Charges(Physics)
Static Charges
Static means stationary, so static charges are stationary charges. First we need to look at atoms to understand static charges
All materials are made up of atom. A diagram of an atom is shown below.
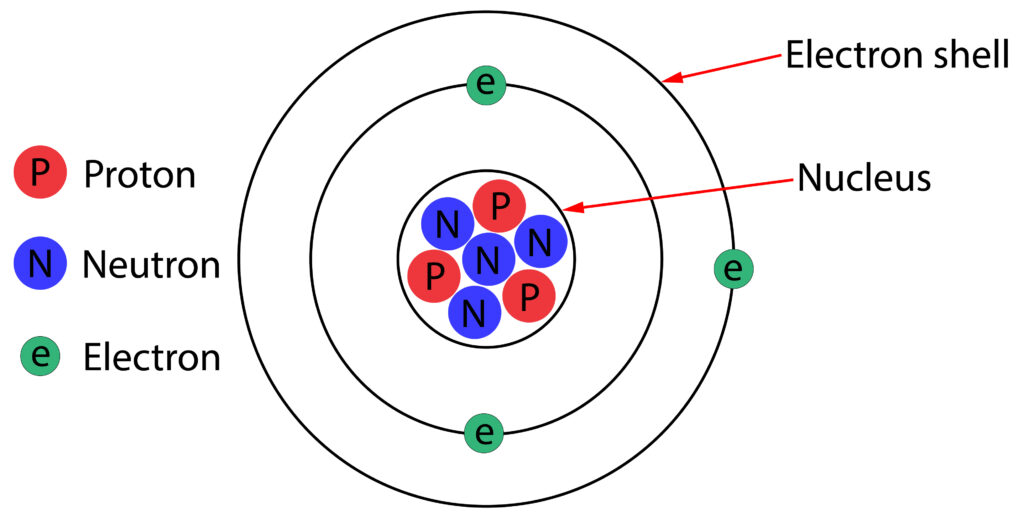
In the diagram above protons have a + 1 charge and electrons have a -1 charge. An atom is electrically neutral because it contains equal numbers of protons and electrons.
Neutrons are neutral, so they do not contribute to the charge of the atom.
Ions
If an atom loses or gains electrons, it will form an ion.
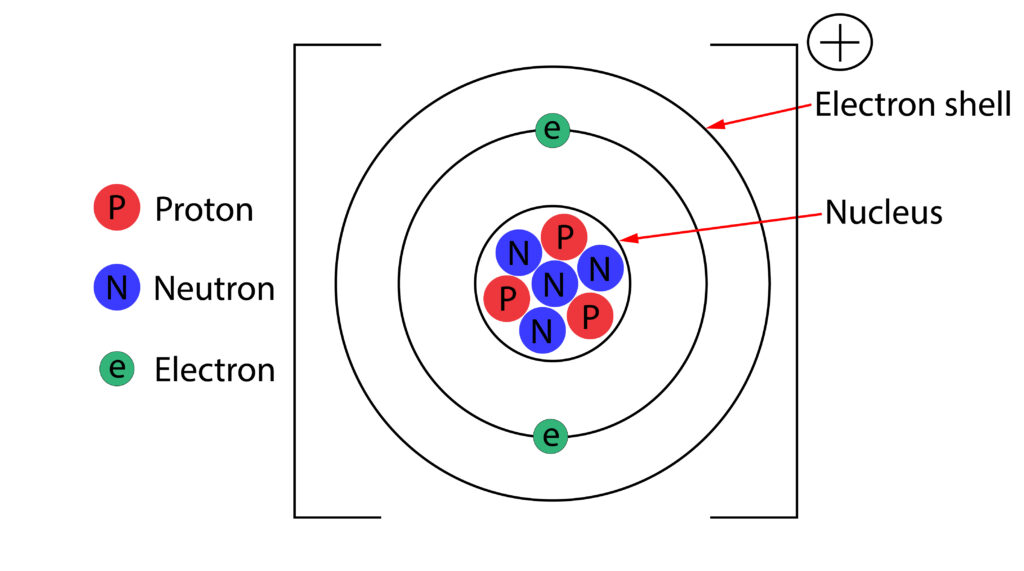
In the diagram above the atom is now a positive ion, because it has lost an electron. There is now one more proton, than electron, so its a +1 ion.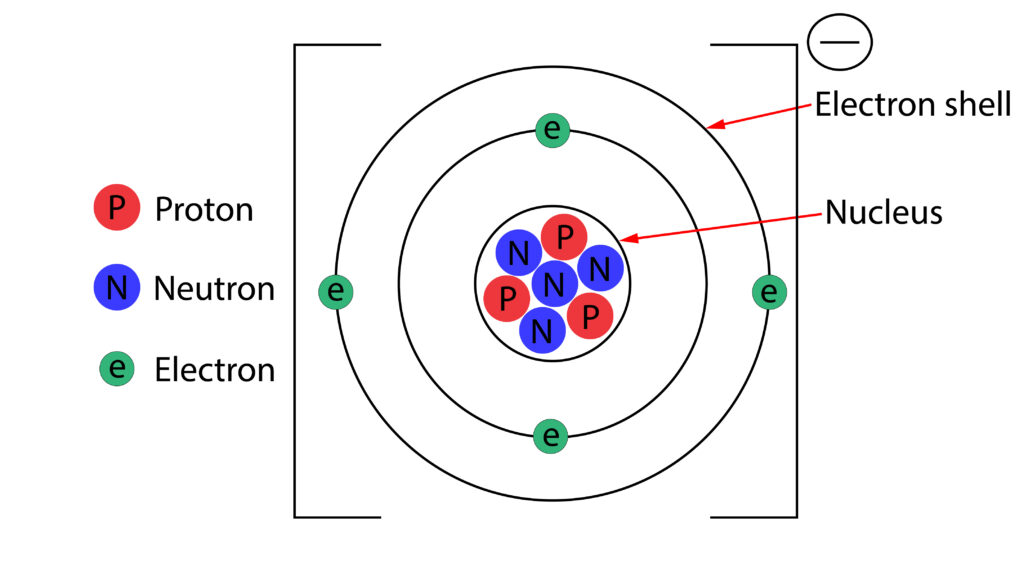
In the diagram above the atom is a negative ion, because it has gained an electron. There is now one more electron than proton, so this is a -1 ion.
Practice Questions
1.Explain why atoms are electrically neutral
2. State the charge on an ion, if the atom loses two electrons
3. An ion contains 7 protons, 10 electrons and 7 neutrons. State the charge on the ion
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque