AQA GCSE Writing nuclear fission equations(Physics)
Writing nuclear fission equations for induced fission.
In induced fission, a neutron will collide with a nucleus and then split into two daughter nuclei, produce 2 or 3 neutrons and release energy.
Lets start with induced fission of U-235, by writing the reactants.

The neutron is on the left, Uranium 235 is adjacent, the atomic number of 92 can be found from the periodic table. Then put an arrow to the right of both of them.
Now we need to write down the daughter nuclei that will be formed to the right of the arrow. The exact nuclei that form are not important, but they should be roughly the same size. I have selected Barium-131 and Krypton-92
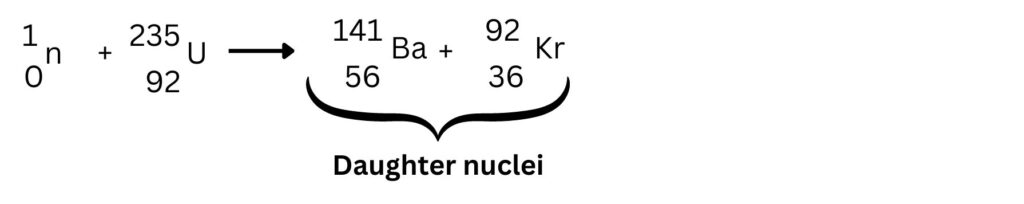
What is important, it to check the atomic numbers. So the 56 and 36 should add up to 92. Remember to look up the symbols, using the atomic numbers.
The mass numbers of 92 and 141 will add up to 233. The U-235 + 1 neutron gives a total mass number of 236. So, we are 3 mass numbers short, this is because we need to add of 3 neutrons being produced. See below:
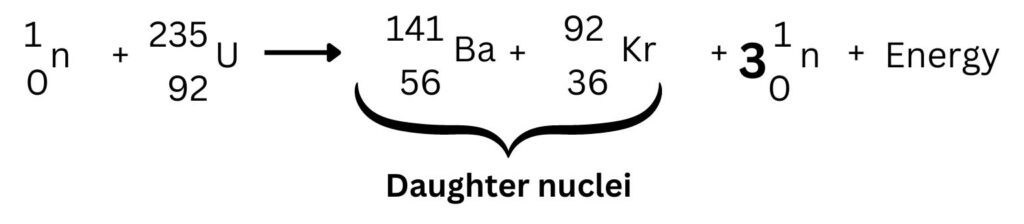
Energy is always released, so energy is just added on. Remember the energy is released, not produced
It is unlikely you will be building these equations from scratch, but you may have to fill in one blank. See the practice questions below
Writing nuclear fission equations for spontaneous fission
In spontaneous fission, the nuclei will split on its own. We will have a look at building the nuclear fission equation for the spontaneous fission of Californium-252. Lets start by putting down the reactants and an arrow.

Now we need to select the daughter nuclei. Again the exact nuclei chosen is not important, but both daughter nuclei should be similar.

It is important to check the atomic numbers of the daughter nuclei that they add up to 98, in this case 55 + 43 do add up to 98.
The 140 +109 add up to 249. This is 3 mass units short of 252 because 3 neutrons are produced during the equation.

Practice Questions
1. Complete the following induced fission equations

2. Complete the following spontaneous fission equations

Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque
Accordion Content