AQA GCSE Specific Latent Heat(Physics)
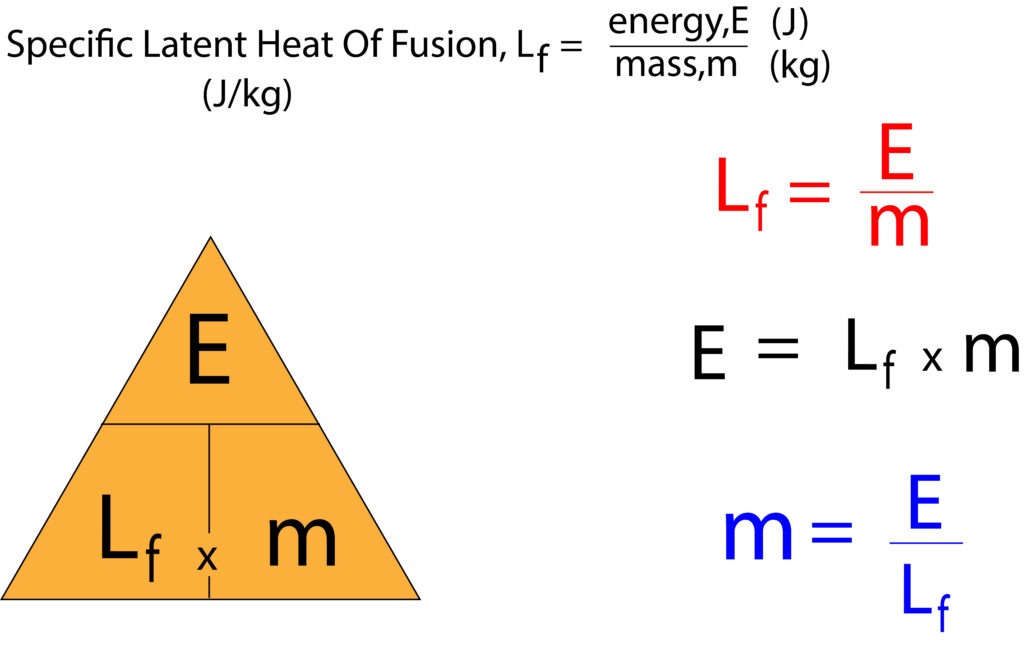
Specific Latent Heat
Specific latent heat involves the amount of energy needed to change the state of 1kg of material, without changing the temperature.
We can be more specific though (LOL):
Two types of specific latent heat:
1.Specific latent heat of fusion which is the energy needed to change the state of 1kg of a solid to a liquid at its melting point, without changing the temperature.
2.Specific latent heat of vaporisation is the energy needed to change the state of 1kg of the substance from a liquid to a vapour at its boiling point, withough changing the temperature.
The diagram below will show how to measure specific latent heat of fusion
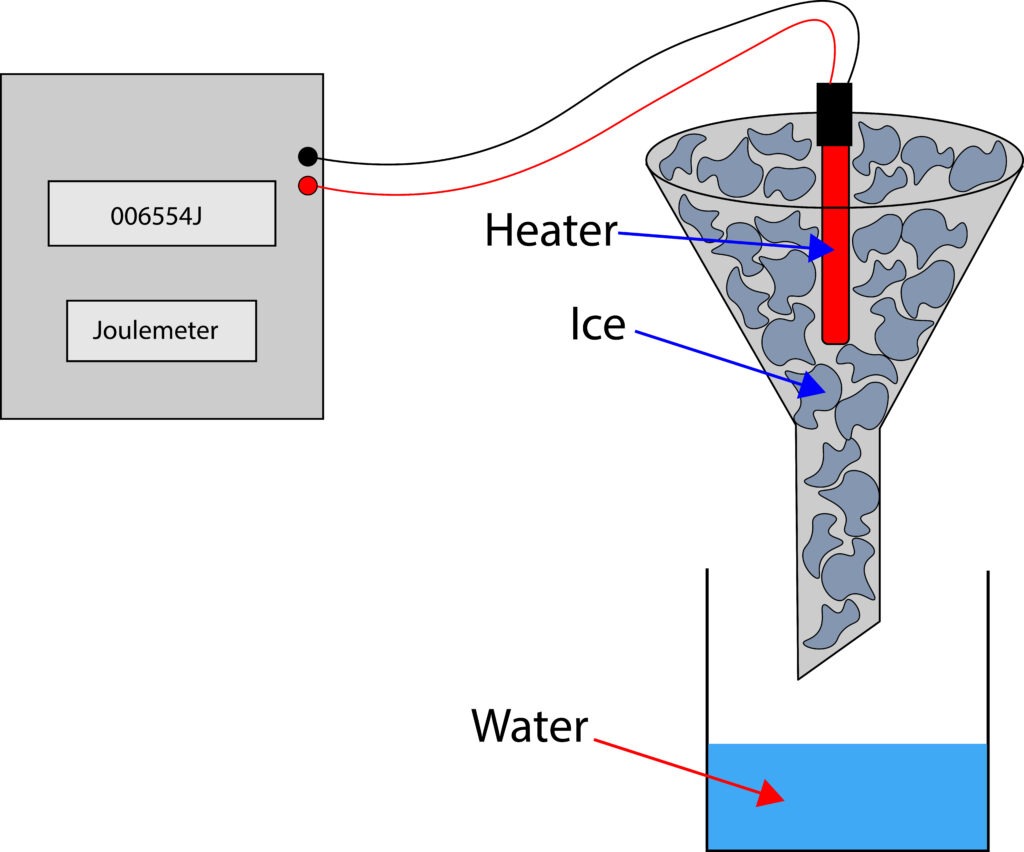
First we need to find the mass of ice that will melt due to room temperature. So, with the heater switched off, the ice is allowed to melt and water collected in the beaker for 5 minutes. The mass of the beaker and water are recorded e.g. 120g.
Now the experiment is repeated for the same time period, but with the heater switched on for 5 minutes. Readings of the joulemeter are recorded at the start of heating and once heating is completed. After heating the mass of the beaker and water are recorded e.g. 170g.
If we subtract these masses, it will give the mass of ice that melts due to the heat energy being supplied.
Mass of ice that melts due to heat energy being supplied 170g-120g = 50g or 0.05kg
Joulemeter reading before = 006452 J
Joulemeter reading after = 023252 J
Energy supplied = 023252-6452 = 16800J
Using the formula above Lf = E/m
Lf = 16800/0.05 = 336000 J/kg
Practice Questions
1. Explain the difference between specific latent heat and latent heat.
2. In the experiment above why is the heater switched off for the first part of the experiment
3. Substance X has a specific latent heat of fusion value of 290000J/kg and it needs 50,000J to change its physical state from a solid to a liquid. Calculate the mass of substance X.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque
Accordion Content