AQA GCSE Radiation types and properties(Physics)
Radiation types
There are four types of radiation
1. Alpha particle
2.Beta Particle
3. Gamma Radiation
4. Neutron
Alpha Particle
 An alpha particle is made up of a helium nucleus, containing 2 protons and 2 neutrons.
An alpha particle is made up of a helium nucleus, containing 2 protons and 2 neutrons.
Alpha particles have low penetration, travelling only a few cm in air. They are stopped by other materials such as paper.
Alpha particles have a relative charge of +2, due to the 2 protons, so they are highly ionising
Beta Particle
 A beta particle is an electron.
A beta particle is an electron.
During a beta decay a neutron turns into a proton and a high speed electron is released from the nucleus.
Beta particle has a relative charge of -1, so it is moderately ionising.
Beta particles have moderate penetration, so they can travel through paper, but are stopped by a few mm of aluminium.
Gamma Radiation
 Gamma Radiation is an electromagnetic wave. The nucleus of the atom emits gamma electromagnetic radiation.
Gamma Radiation is an electromagnetic wave. The nucleus of the atom emits gamma electromagnetic radiation.
Gamma is uncharged, so it is the least ionising when compared to alpha and beta.
Gamma has high penetration power, virtually unlimited range in air, it can pass through a few mm of aluminium, but is stopped by thick lead sheets or thick concrete.
Penetration Power

Ionising ability
Radiation that is ionising has enough energy to knock electrons off of atoms to form ions.
Alpha has highest charge, so it is the most ionising. Gamma is uncharged, so it is the least ionising. Beta is in the middle as its moderately ionising.
Using Data from Radioactivity Experiments
To do well, you need to be able to apply the information and use data
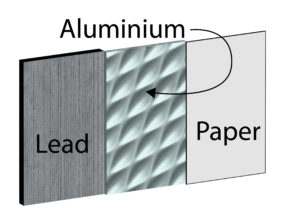
A teacher placed this absorber between a radioactive source and a Geiger Muller tube to detect the radiation. The absorber contains 3 materials Lead, Aluminium and Paper.
These were the data values that the teacher recorded:
| Absorber | Count rate per second |
|---|---|
| No absorber (air only) | 5100 |
| Paper | 4960 |
| Aluminium | 350 |
| Lead | 30 |
Using the information in the table above suggest the radioactive source present
It is not Alpha, because the count rate is still high after it passes through the paper
It is not gamma because the count rate decreases significantly through the aluminium.
Therefore, it must be beta as the count rate does not decrease significantly through the paper, but it does decrease a lot through the aluminium.
Neutrons
A neutron is a sub atomic particle normally found in the nucleus.
It has a mass, but no charge.
Neutron emission can occur in nuclear reactors. where neutrons are released through nuclear fission.
It can also occur when an alpha particle hits a nucleus such as Beryllium-9 making it unstable, so it undergoes decay to form carbon-12 and emits a neutron.
You don’t need to know about penetration or ionisation of neutrons. However, neutrons tend to be quite penetrating and due to having no charge they are not particulary ionising.
Practice Questions
1.State the names of the four types of radiation that can be emitted from a radioactive source
2. State the composition of an alpha particle
3. Why is an alpha particle highly ionising?
4. How far can an alpha particle travel in air?
5. State the composition of a beta particle
6. How is a beta particle formed
7. State the name of a metal which will stop beta particles travelling through it.
8. Why is gamma considered to be less ionising than alpha or beta?
9. Which materials can stop gamma radiation?
10. Explain the term ionising.
11. When radiation is passed through paper the count rate significantly decreases. Suggest the type of radiation present.
12. A radioactive source transmits radiation easily through paper and aluminium, but the count rate decreases through lead. Suggest the type of radiation present
13. State two ways that neutrons can be emitted.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque