AQA GCSE Gas pressure and volume(Physics)
Gas Pressure
Gas pressure is caused by moving gas particles colliding with the walls of the container and exerting a force. This force exerted is gas pressure.
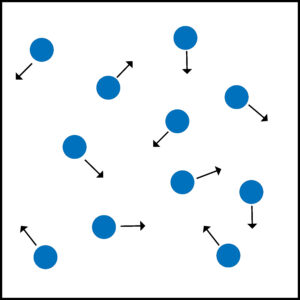
As the gas particle collides with the wall of the container a net force is exerted at 90 degrees to wall of the container.
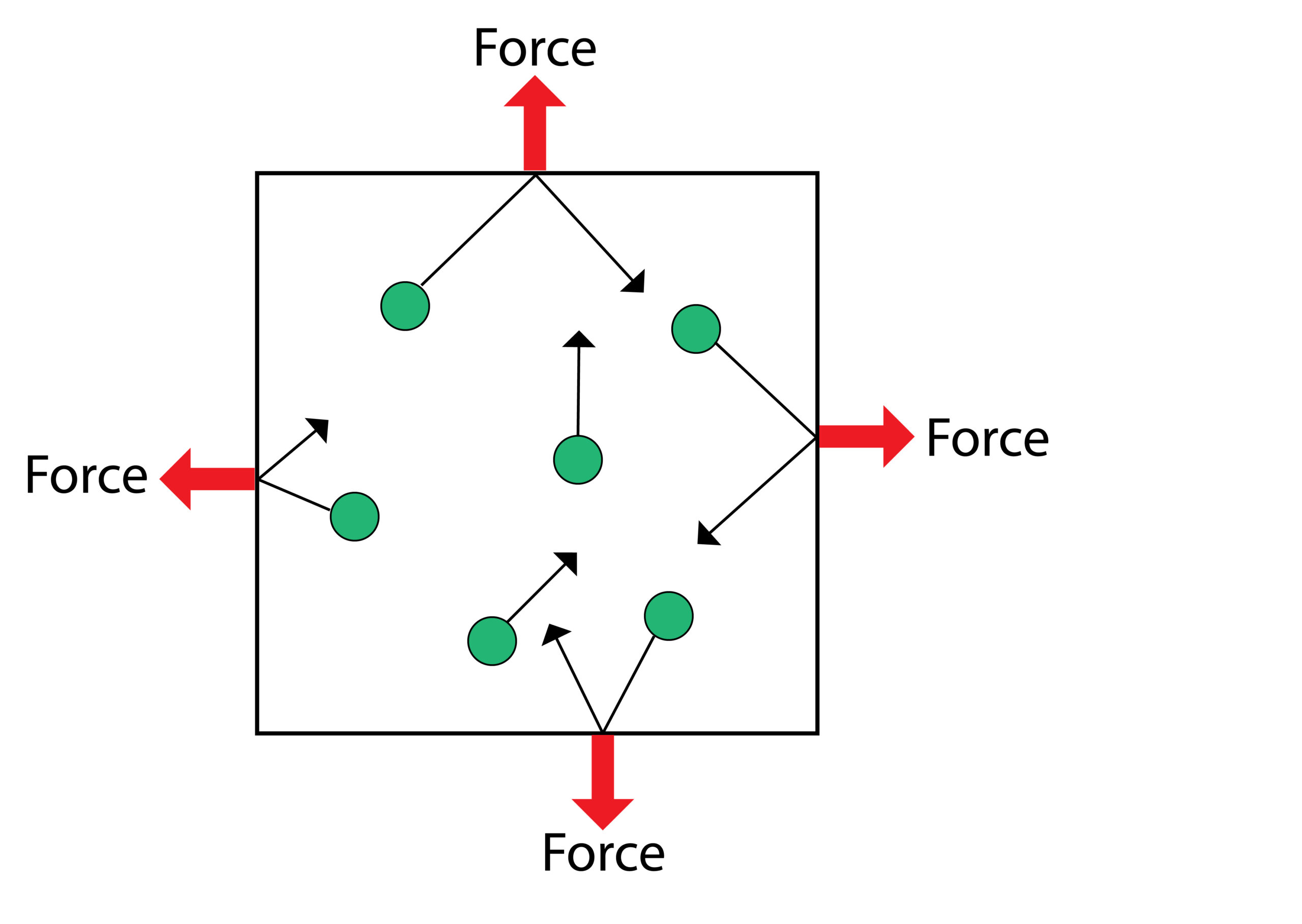
Gas Pressure and Volume
There is a lot of space between the particles of a gas, so gases can be compressed.
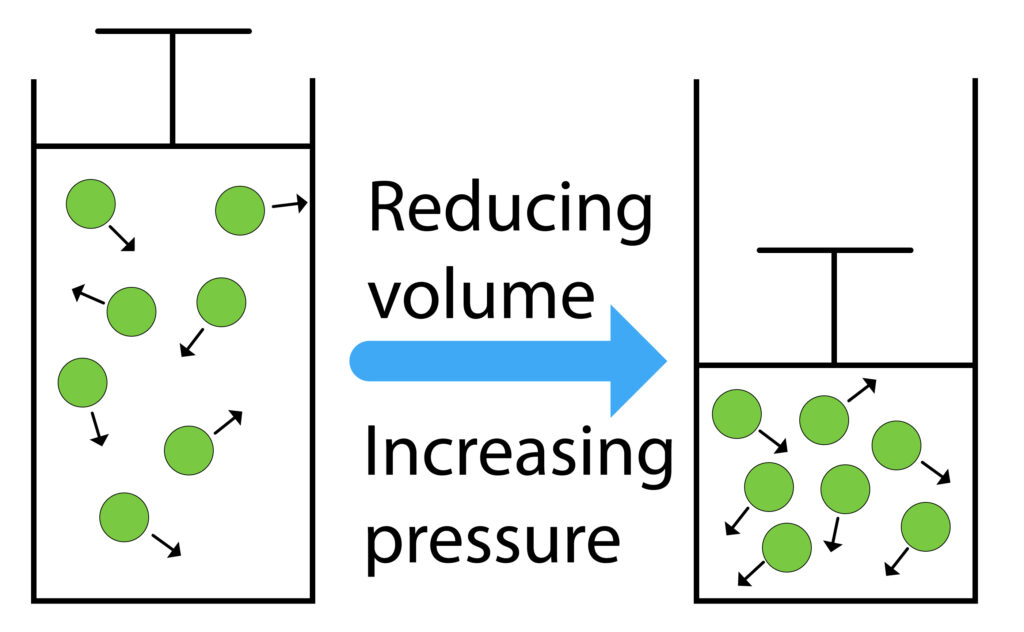
When the plunger is pushed in the volume of the container will decrease, this will increase the pressure.
When the volume of the container is smaller, the gas particles collide with the walls of the container more frequently, so gas exerts a larger net force, so gas pressure increases.
Generally:
Pressure increases, as the gas is compressed in a smaller container
Pressure decreases as the gas expands in a larger container.
Boyles Law
Pressure and volume are inversely proportional to each other. This means that as Volume increases, pressure will decrease.
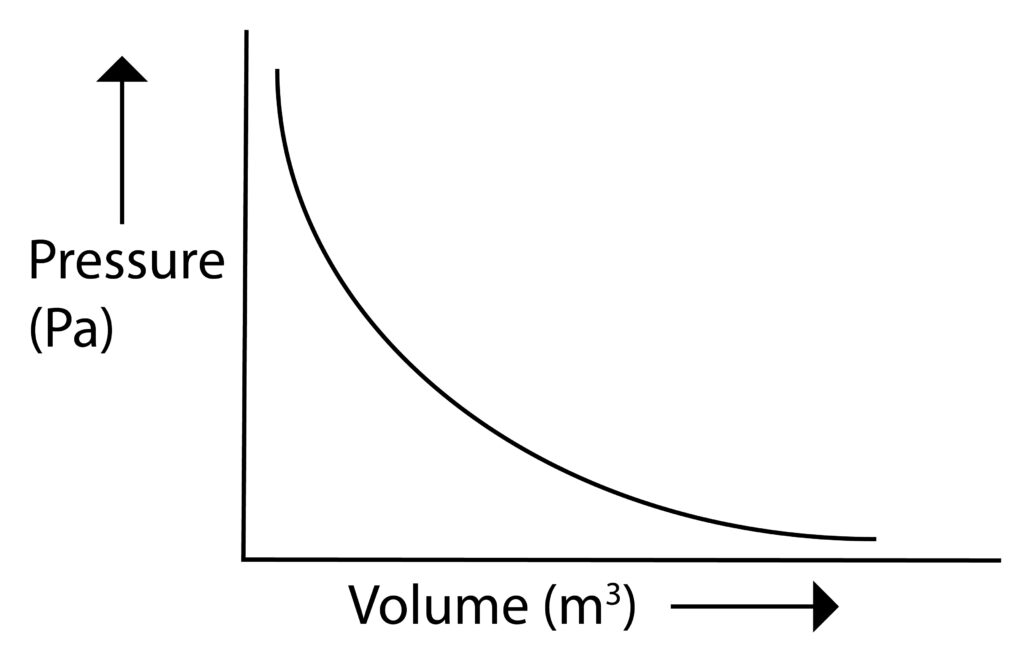
If there is a fixed mass of gas at constant temperature then:
Pressure,P x Volume,V = Constant
This equation can be rewritten as:
Pressure1,P1 x Volume1,V1 = Pressure2,P2 x Volume2,V2
P1 xV1 = P2 x V2
P1 = Initial pressure (Pa)
P2 = Final pressure (Pa)
V1 = Initial volume (m3)
V2 = Final volume (m3)
A gas has a volume of 100m3 and a pressure of 20Pa. It is compressed to occupy a volume of 40m3. Calculate the new pressure.
Initial pressure P1 = 20Pa
Initial Volume V1 = 100m3
Final volume V2 = 40m3
Final pressure P2 = ?
See the video below for workings and solution
1. Explain how gas pressure is caused.
2. State the relationship between gas pressure and gas volume
3. Describe the appearance of a graph that has gas pressure on the Y axis and 1/volume on the X axis
4. Gas Y is compressed down to a volume of 20m3 with a gas pressure of 50Pa. The original gas has a pressure of 15Pa. Calculate the original gas volume before compression.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque