AQA GCSE Ernest Rutherford and Nuclear Model(Physics)
Ernest Rutherford and Nuclear Model
Ernest Rutherford asked two of his students Geiger and Marsden to carry out an experiment where they fired alpha particles at thin gold foil to probe the structure of the atom.
As a result of this alpha particle experiment the previous plum pudding model proposed by J.J Thomson was discarded.
Alpha Particle scattering Experiment
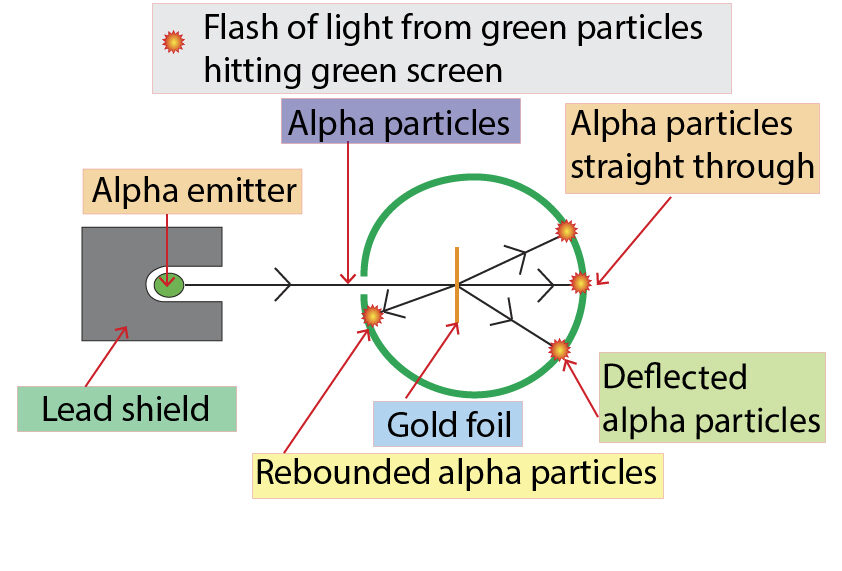
Rutherford was surprised by the results of the experiment.
When the alpha particles reached the gold foil one of three events occured.
1.Most of the alpha particles went straight through the gold foil, which suggests that most of the atom is empty space.
2. Some of the alpha particles went through the gold foil, but were deflected slightly. See the line in blue below.
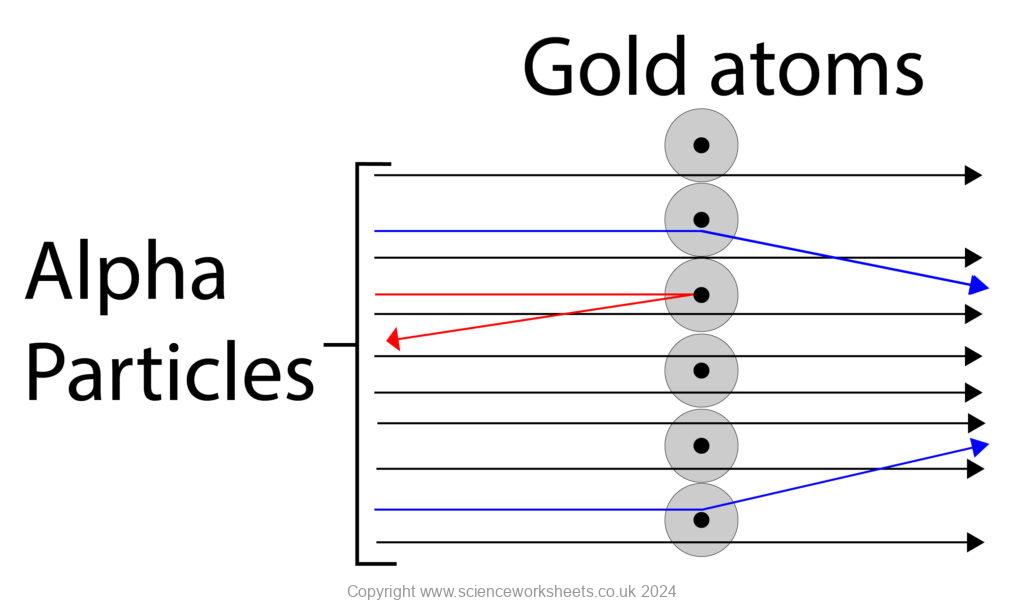
Both the alpha particles and the nuclei of the gold atoms are positively charged. Like charges repel each other, so the alpha particles were pushed away from the gold nuclei, causing them to change direction. This shows that the nucleus of the atom is positively charged.
3. One or two alpha particles bounced back, shown by the red line in the diagram. This rare event happened because an alpha particle hit a nucleus. This shows that the nucleus is very small compared to the size of the atom, and that most of the atom’s mass is in the nucleus.
Replacing the Plum pudding model with the Nuclear model.
Under the plum pudding model, the alpha particles should had gone straight through the gold foil, with no rebound.
However, to Rutherford’s suprise some of the alpha particles rebounded, so the plum pudding model was then replaced by the nuclear model.
Nuclear Model:

Practice Questions
1. Describe how the alpha particle scattering experiment was carried out
2. During the alpha particle experiment, several events were seen. State what each of the following events tells us about the structure of the nucleus
2a.Most of the alpha particles went straight through the gold foil
2b. Some of the alpha particles were deflected as they passed through the gold foil
2c.One or two alpha particles rebounded when they hit the gold foil
3.Based on the plum pudding model, what should had happened to the alpha particles on reaching gold foil
4.What was the name of the model, which then replaced the plum pudding model.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque