AQA GCSE Discovery of the Proton and Neutron(Physics)
Nucleus
At the time the structure of the nucleus was unknown, so it was treated as a positively charged sphere, its exact structure was discovered later.
Discovery of the proton
Ernest Rutherford discovered the proton.
Around 1917 he bombarded nitrogen nuclei with alpha particles and this led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles.
Each particle having the same amount of positive charge. The name proton was given to these particles.
Discovery of the Neutron
James Chadwick carried out experimental work to prove the existence of neutrons. This was around 1932, 20 years after the discovery of the nuclear model.
Scientists knew that the mass of a hydrogen nucleus was 1 and its charge was +1 because it contained 1 proton.
Then they looked at other nuclei and the mass was always bigger than the positive charge.
A Lithium nucleus has a mass of 7, but a total positive charge of +3 due to 3 protons. So, another particle was causing this extra mass that had no charge!
This other particle was the neutron!
James Chadwick discovered that the neutron has no charge, but its mass is similar to that of a proton.
As a result the nuclear model was changed again to look like what we know today
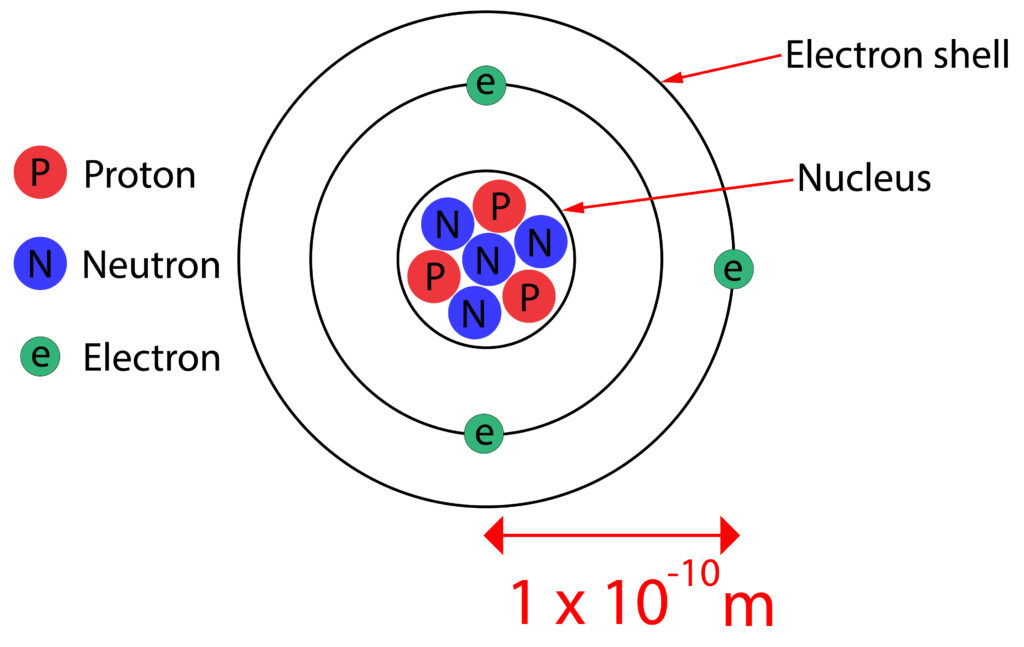
Practice Questions
1.Describe how the idea of protons came about
2.Describe how scientists knew that there were particles other than protons in the nucleus?
3. Which famous Scientist discovered the neutron?
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque