AQA GCSE Calculating Spring Constant(Physics)
Spring Constant
Spring constant is a measure of the stiffness of a spring.
Spring constant,k has units of N/m
This means that it represents the amount of force needed to change the length of a spring.
The higher the value for the spring constant, the stiffer the spring, so the more force that needs to be applied to change the length of the spring.
Formula for Spring Constant
Learn the formulae below:
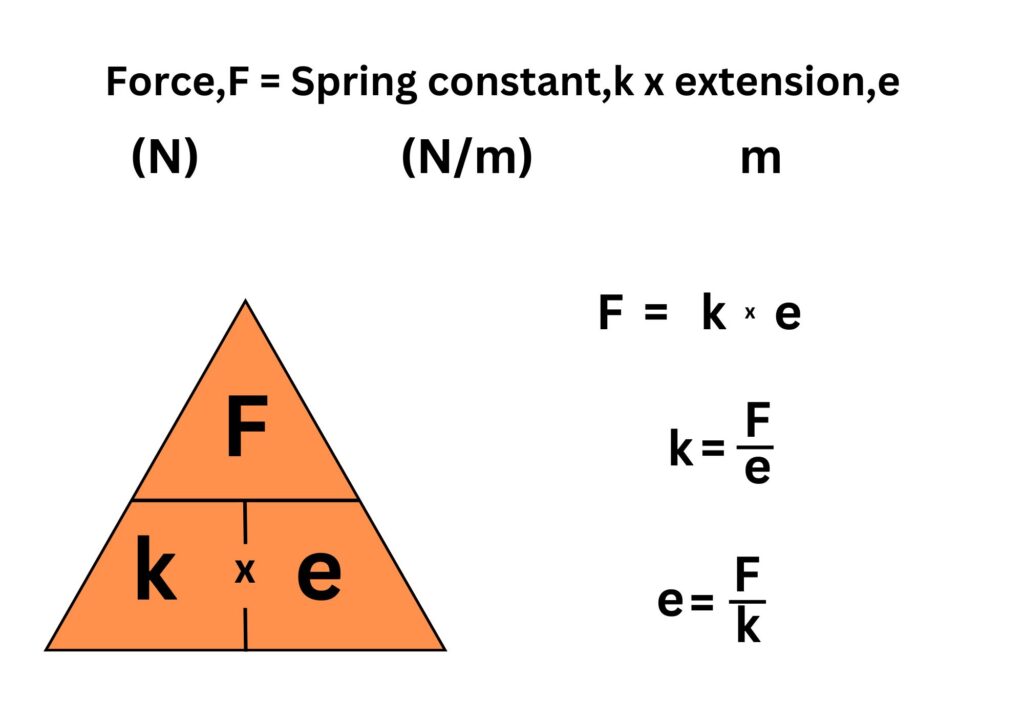
Extension is the change in length.
Example questions
1.A spring has a spring constant of 4N/m and its length is increased by 0.1m. Calculate the force applied
F = ke
F = 4N/m x 0.1 = 0.4N
2.A force of 50N is applied to an object to increase its length by 0.25m. Calculate the spring constant.
k = F/e
k= 50N/0.25m
k = 2N/m
3. The initial length of a spring is 0.2m and it is stretched where its new length is 0.5m. The spring has a spring constant of 30N/m. Calculate the force needed to increase the length of the spring
Extension = New length – Initial length
Extension = 0.5m-0.2m = 0.3m
F = ke
F = 30N/m x 0.3m = 9N
4. A force of 60N is applied to a spring to decrease its length from 70cm to 50cm. Calculate the value for the spring constant
First convert the units for length into m
70cm = 0.7m
50cm = 0.5m
Extension = new length – original length
Extension = 0.5m-0.7m = -0.2m
Although the extension is -0.2m, you can treat it as 0.2m.
k = F/e
k = 60N/0.2 = 300N/m
Practice Questions
1.A material has a spring constant of 100N/m and a force of 25N is applied to increase its length. Calculate the extension.
2.A spring is stretched from 5cm to 30cm and it has a spring constant of 40N/m. Calculate the force needed to stretch the spring
3. A spring is stretched by 50cm using a set force, this spring is replaced with another spring that has a value for its spring constant that is double the previous spring. Assuming the same force is applied, calculate the new extension.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque