AQA GCSE Atmospheric Pressure(Physics)
Earth’s atmosphere
The Earth has a atmosphere which surrounds it, this is a thin layer of air.
The Earth’s atmosphere is about 2% of the thickness compared to the Earth. It can be seen above with the dark blue circle that surrounds the Earth in the image above.
Atmospheric Pressure
As the atmosphere is a layer of gas, atmospheric pressure is caused the same way that gas pressure is caused.
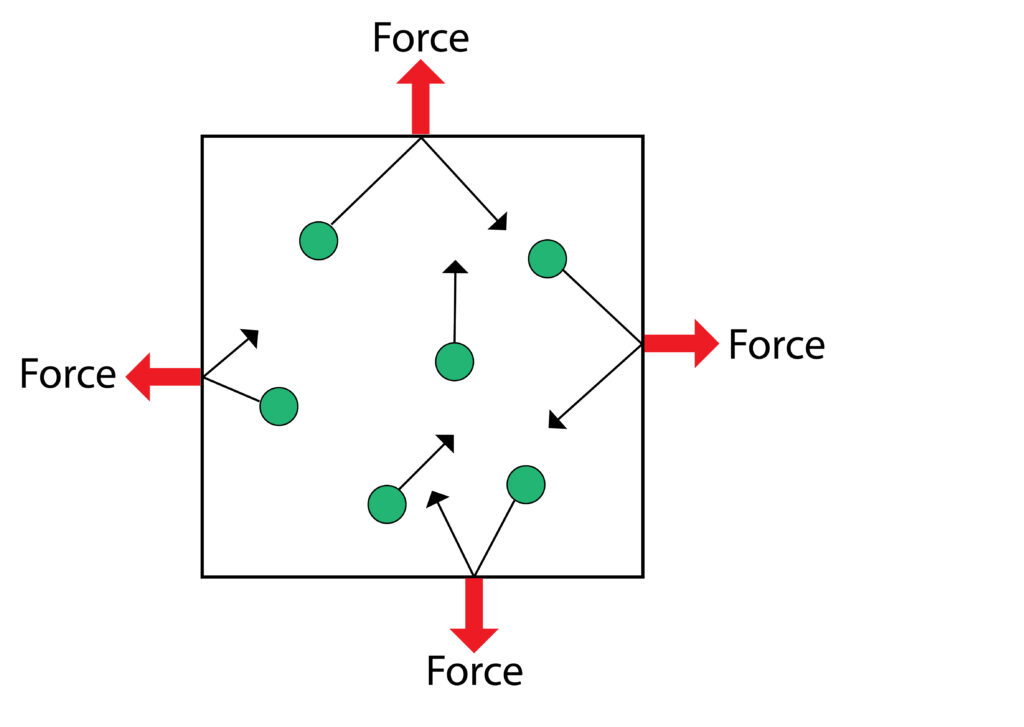
The gas particles in the atomsphere have energy, so they move in random directions, when they collide with a surface, they will exert a force. This force is the atmospheric pressure.
All the time, gas particles in the atmosphere are colliding with objects such as people, buildings, cars etc. So, all of these objects will experience atmospheric pressure.
Density of the atmosphere and altitude.
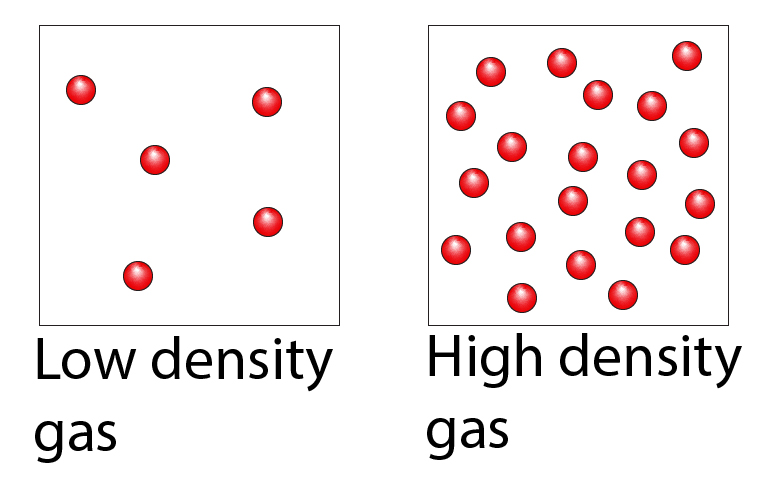
Density is the amount of mass per unit volume. Below are two images which show a low density gas and a high density gas.
The density of the atmosphere will change with altitude as shown below:
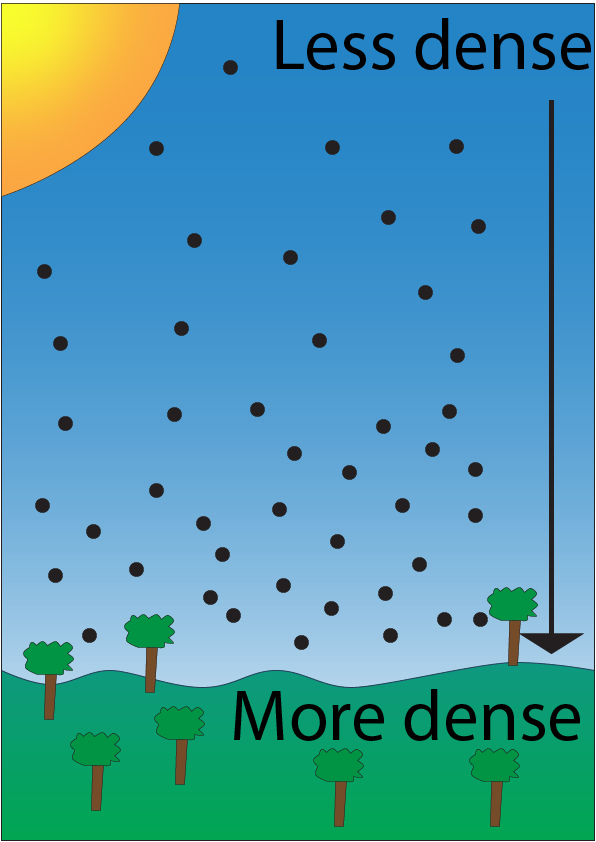
Each black circle represents an air particle. As altitude increases, density of the atmosphere will decrease.
Relationship between atmospheric pressure and altitude
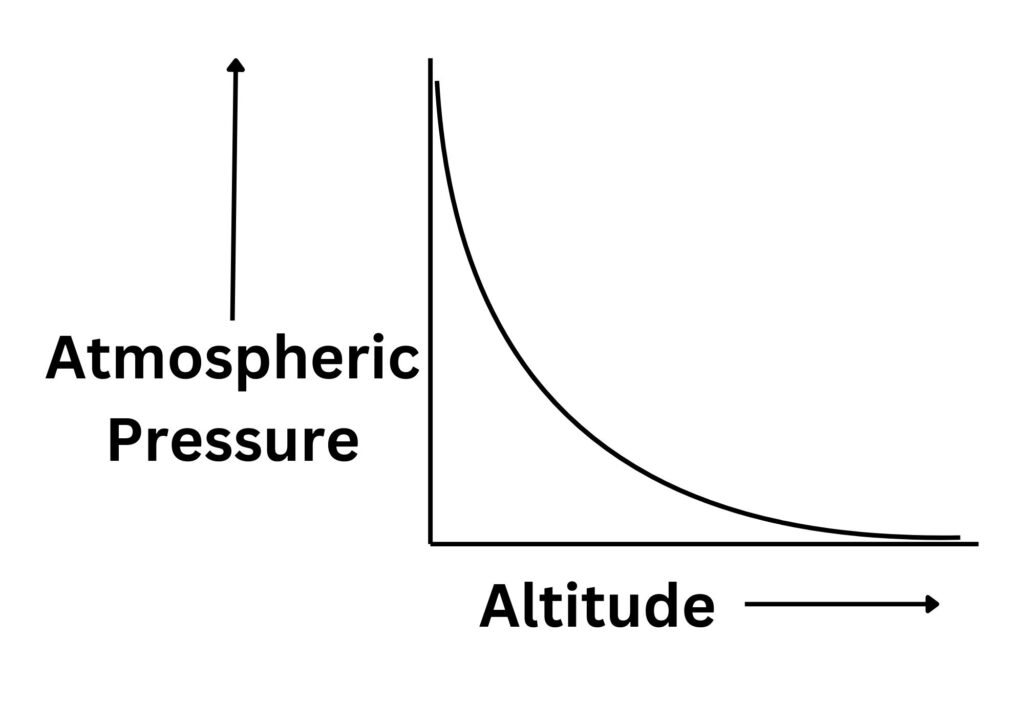
There is an inverse proportional relationship between altitude and atmospheric pressure.
Why does atmospheric pressure decrease with altitude?
As the altitude increases the air becomes less dense. This means that:
1.There is less weight of gas particles above pushing down.
2. Fewer molecules per unit volume and so fewer collisions per unit time
So, atmospheric pressure will decrease with increasing altitude.
Practice Questions
1. Explain how atmospheric pressure is caused
2. Why does the atmosphere become less dense with altitude?
3. State the name for the type of relationship between atmospheric pressure and altitude.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque