Answers to AQA GCSE Half Life Calculations(Physics)
Half Life Calculations
This formula can be used for some of the count rate calculations
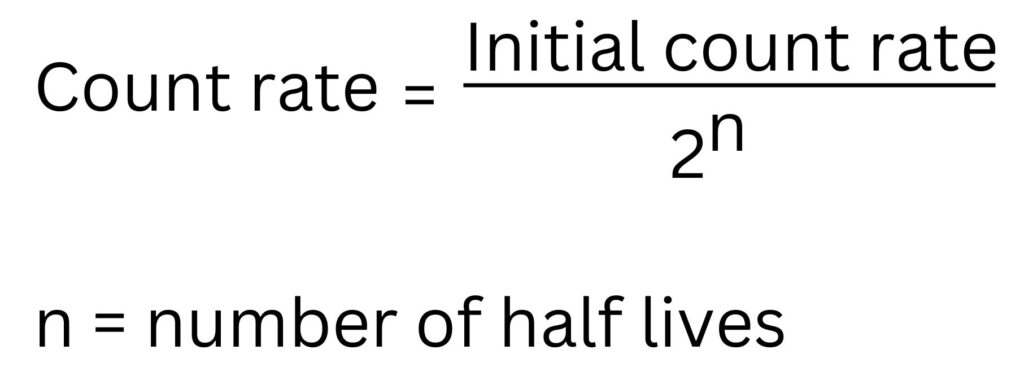
1. Polonium-210 has a half life of 140 days. If an original sample of Polonium-210 has an activity of 500Bq what will its activity be after 560 days.
560 days/140 days = 4 half lives
Count rate = Initial count rate/2n
Count rate = 500/24
Count rate = 31.25
2. A radioactive isotope undergoes a series of half lives. At the start there are 8 x 1036 atoms of the isotope present. At a point later in time there are 1 x 1036 atoms remaining. Calculate the net decline.
Final number of radioactive atoms :Initial number of radioactive atoms
net decline = 1 x 1036 : 8 x 1036
net decline = 1:8
net decline = 1:8
3. If the initial count rate is 200,000Bq. Calculate the net decline after 4 half lives
The count rate will need to be halved 4 times
200,000/2 = 100,000Bq
100,000/2 = 50,000Bq
50,000/2 = 25,000Bq
25,000/2 = 12,50Bq
So, after 4 half lives the count rate is 12,500Bq
net decline = Final count rate:Initial count rate
net decline = 12500:200000
net decline = 125:200
net decline = 25:40
net decline = 5:8
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque