AQA GCSE Nuclear Fusion(Physics)
Nuclear Fusion
Nuclear fusion is the joining of two light nuclei to form a heavier nucleus.

Nuclear fusion occurs naturally in stars such as the Sun. Our Sun fuses hydrogen nuclei to form Helium nuclei, releasing energy. Our Sun is approx 4.5 billions years old and is about half way through its life cycle.
Nuclear Fusion of Hydrogen nuclei to Helium nuclei
In the simplified diagram below two hydrogen nuclei are fusing to form helium. In real life, this is more complicated. However, you do not need the more complicated version for GCSE.
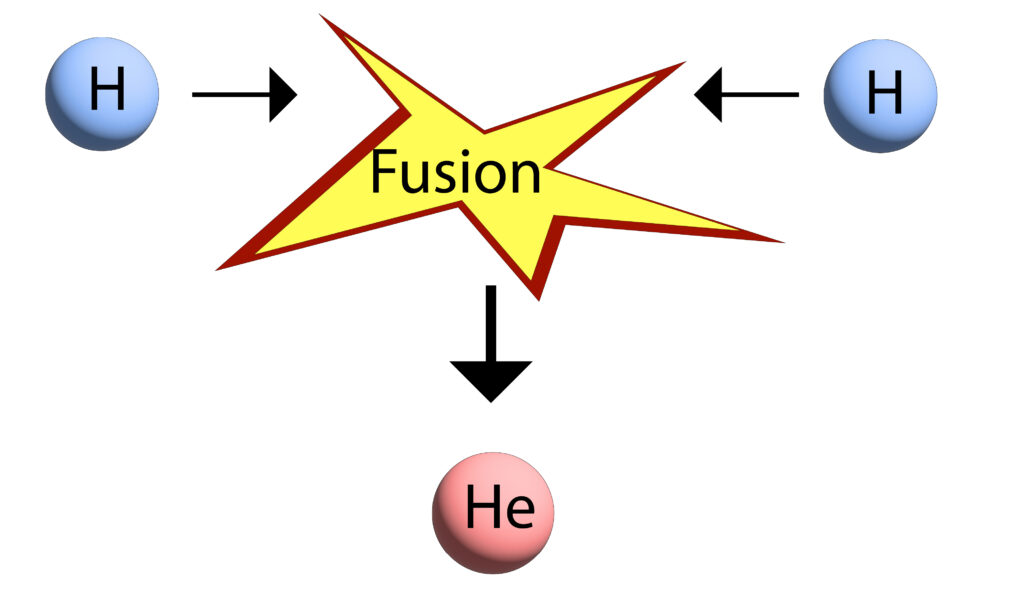
During the process some of the mass is converted to energy. This is why the Sun emits both light and thermal energy, which we receive on Earth.
Energy released through nuclear fusion
During any nuclear fusion reaction, there is a change in mass. This change in mass relates to a change in energy.
Einstein’s famous equation can be used to calculate the amount of energy.
Energy = Mass x Speed of light2
(J) (kg) (m/s)
The speed of light is 3 x 108m/s. So, when this quantity is squared, it forms a very large number. Then this is multiplied by the mass to find the energy. So, even from a very small mass a large amount of energy is released.
Can we use nuclear fusion on Earth to release energy?
Nuclear fusion releases a lot of energy, from a small mass. However, it is hard to carry out nuclear fusion on Earth.
If we look at the previous example of the hydrogen nuclei fusing to form helium. Both hydrogen nuclei are positively charged, so they repel. In stars there are huge gravitational fields that can force the nuclei together to overcome the repulsion.
On Earth in a nuclear fusion reactor it is difficult to overcome this repulsion. It needs very high temperatures to make the nuclei fuse. At the moment nuclear fusion reactors are mostly experimental.
Nuclear fusion reactors would be a development for the future.
Practice Questions
1. State the definition of nuclear fusion
2.Explain the origin of energy that is released from stars
3. Why do experimental fusion reactors currently use more energy than they can release?
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque