AQA GCSE Specific Heat Capacity(Physics)
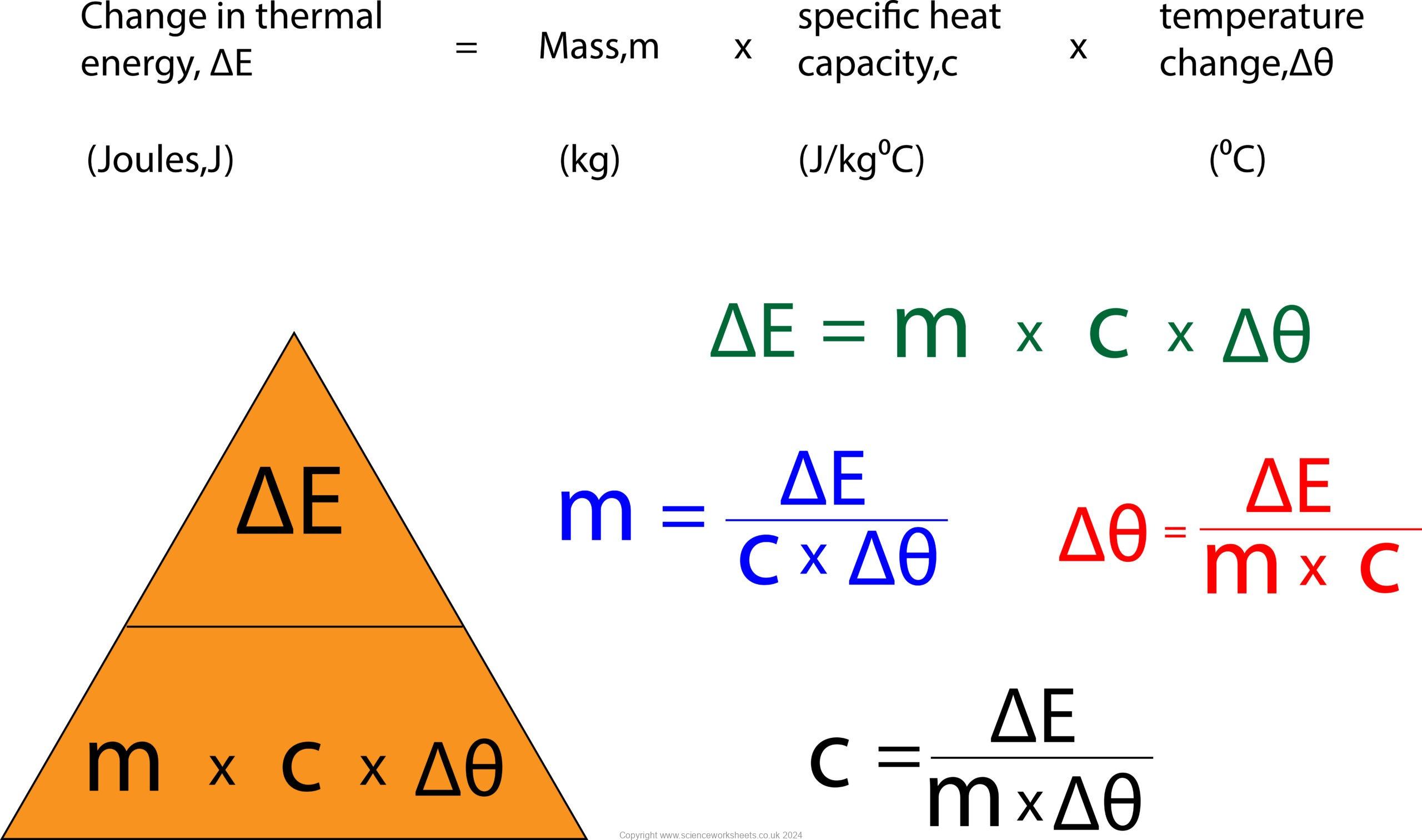
Specific Heat Capacity
The specific heat capacity of a substance is the amount of energy required to raise the temperature of one kilogram of the substance by one degree Celsius.
Why the value of specific heat capacity matters
As the value for specific heat capacity increases more energy is needed to raise the temperature of a certain mass of substance.
Good conductors such as metals have low specific heat capacity. Only a small amount of energy is needed to raise the temperature of a metal
Poor conductors such as water have a high specific heat capacity, so a lot of energy is needed to raise the temperature of water. This is why the ocean is cold around the UK!
Water is used as the fluid is central heating systems because it can store a lot of thermal energy . So, the water in the radiator stays hotter for longer, allowing more energy to be transferred to the thermal energy store of the room.
Example Questions
Use the following data to help you to answer the questions.
| Name of Substance | Specific heat capacity (J/kg⁰C) |
|---|---|
| Aluminium | 900 |
| Marble | 880 |
| Gold | 130 |
| Steel | 425 |
| Water | 4200 |
1.A gold ring is heated by supplying 1000J of thermal energy to raise its temperature from 25ºC to 100ºC. Calculate the mass of the ring
m = ΔE/(c x Δθ)
Δθ = 100-25 = 75°C
m = 1000/(130 x 75)
m = 0.10 kg
2. A steel drum of mass 20kg contains 30kg of water. Calculate the energy required to raise the temperature of both by 10ºC
ΔE = m x c x Δθ
Energy change for steel drum
ΔE = 20 x 425 x 10 = 85,000J
Energy change for water
ΔE = 30 x 4200 x 10 = 1,260,000J
Total energy is found by adding these together
ΔE(total) = 1,260,000+85,000 = 1,345,000J
3. When a home central heating system is first switched on it takes a while for the radiators to become hot. Explain why it takes a long time for the temperature of the radiators to increase.
Water has a high specific heat capacity. This means that a lot of energy needs to be supplied to raise the temperature of water. Therefore, it takes a while for the water and radiator to reach a high temperature.
Practice Questions
Use the table above to answer the following questions
1. A marble statue of mass 2.5kg receives 10kJ of thermal energy from the Sun. Calculate the increase in temperature of the marble statue.
2. An aluminium water container of mass 900g contains 1.5kg of liquid water. The temperature of both increase from 20ºC to 90ºC. Calculate the total energy needed.
3. A new substance has been discovered and has been named Ty. When 1100g of Ty is heated by supplying 6000J of energy its temperature increases by 40ºC. Calculate the specific heat capacity of Ty.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations
Drawing ray diagrams for a concave lens
Drawing Ray Diagram to produce a virtual image for a convex lens
Drawing ray diagram to produce a real image for a convex lens.
Specular and Diffuse Reflection
Seeing Coloured Objects Part 2
Viewing objects through coloured filters
Transparent, Translucent and Opaque