GCSE States of Matter
States of Matter
There are 3 states of matter:
1. Solids
2. Liquids
3. Gases
Solids
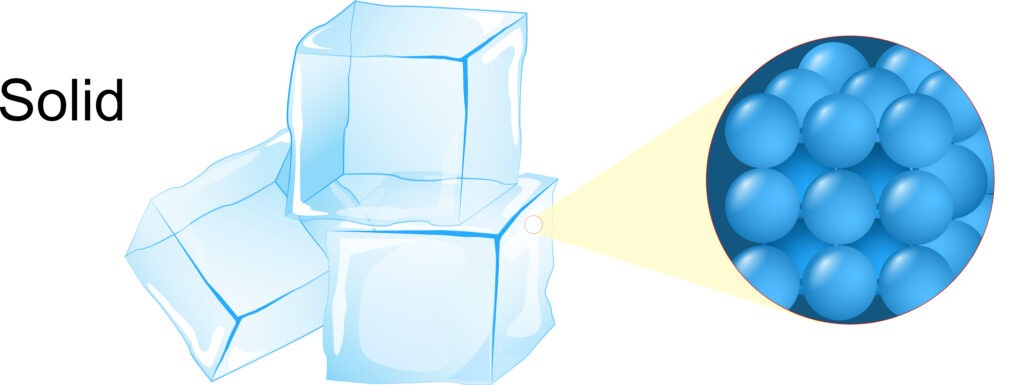
Solids have a fixed shape and fixed volume, they cannot flow.
The particles of a solid are arranged in a regular pattern and are close together.
As the particles are close together, tightly packed solids have a high density. Solids have similar densities to liquids.
Each particle vibrates about a fixed position.
(Never state that particles of a solid move, because they don’t. If you write this it will freak out the examiner!)
Liquids
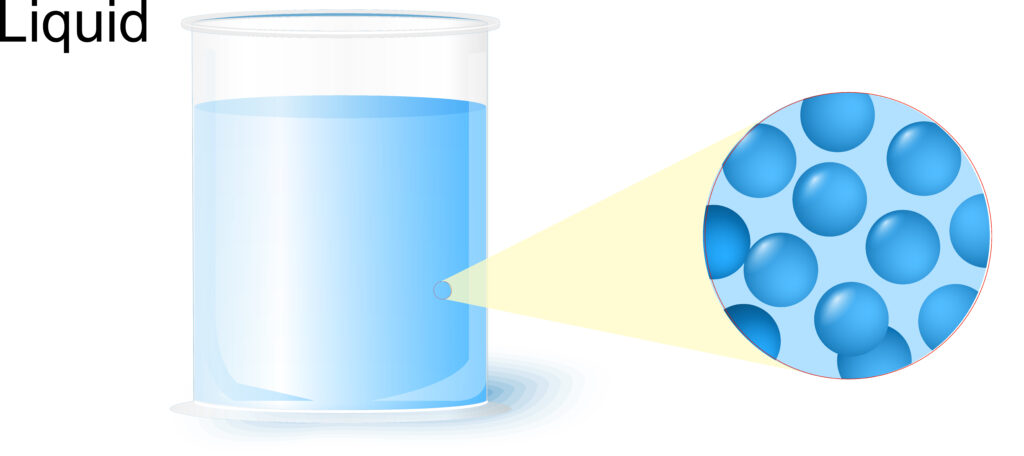
Liquids have fixed volume, but they can take the shape of the container because the particles in a liquid can move and slide over each other.
The particles in a liquid are still close together, but slightly more space between them compared to a solid. As the particles are close together liquids have higher density than a gas. Liquids and solids have similar density.
The particles in a liquid are randomly arranged.
Gases
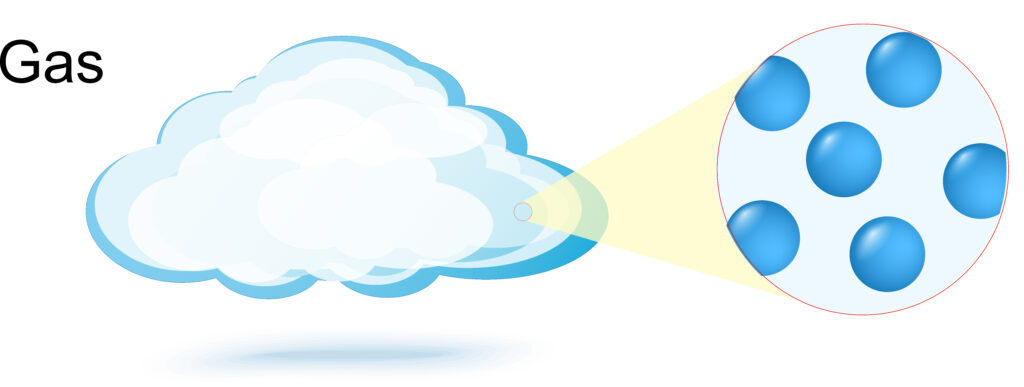
Gases have no fixed shape, they will take the shape of the container.
Gases have no fixed volume if a gas is placed into a container it will expand to fill the container. This is because the gas particles move further apart.
Gas particles are arranged randomly far apart and move in random directions. As there is a lot of space between the gas particles a gas can be compressed, in addition gases have low density.
Gases have lower density than both solids and liquids.
Practice Questions
1.Fill in the table below.
| Property | Solids | Liquids | Gases |
|---|---|---|---|
| Arrangement of particles (Random or Regular) | |||
| Density (High or Low) | |||
| Shape (Fixed or not fixed) | |||
| Volume (Fixed or not fixed) | |||
| Particles (Move or Vibrate) |
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations