GCSE Niels Bohr changing the Nuclear Model
Niels Bohr and the Nuclear Model
Niels Bohr published a series of papers on the model of the atom in 1913. This changed the nuclear model of the atom.
Niels Bohr proposed that:
Electrons orbit the nucleus at specific distances
Below is an image of Niels Bohr proposed model of the atom:
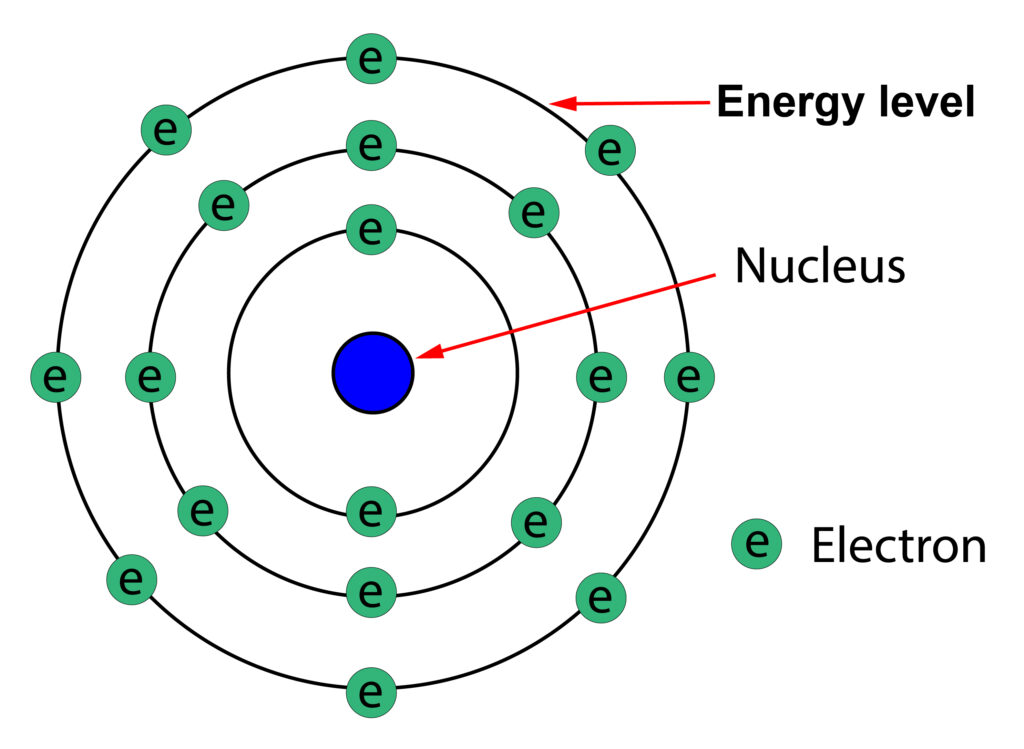
Energy levels
In Niels Bohr model, the electrons are in energy levels at specific distances from the nucleus.
The first energy level can hold up to two electrons.
The second and third energy level can hold up to 8 electrons
How did Niels Bohr come up with this idea?
Niels Bohr carried out experiments and the observations of these experiments matched his theoretical calculations.
You do not need any details of these experiments for your course, but if you wish you can google them.
Nucleus
At the time the structure of the nucleus was unknown, so it was treated as a positively charged sphere, its exact structure was discovered later.
Practice Questions
1. How is the Niels Bohr nuclear model of the atom different to the Rutherford model of the atom?
2. How did Niels Bohr know about the energy levels?
3. What did Niels Bohr propose about the nuclear model?
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations