GCSE Latent Heat
Latent Heat
Latent heat is the energy needed to change the state of a substance.
Latent heat of fusion is the energy needed to melt a substance
Latent heat of vaporisation is the energy needed for boiling a substance
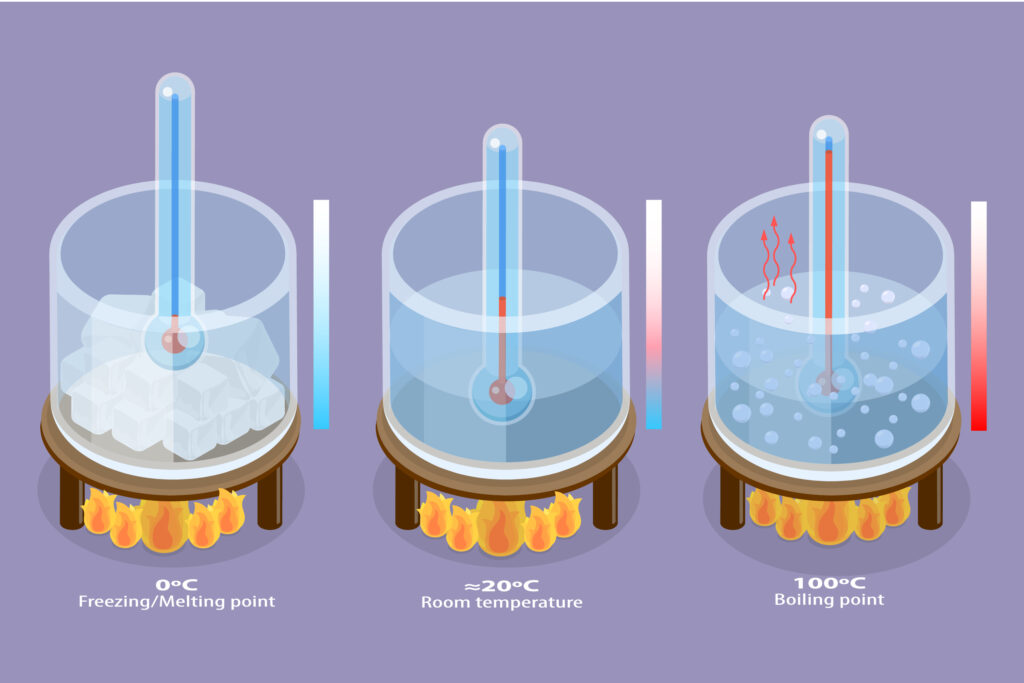
In the experiment below the substance is heated continuously, so the ice melts to form liquid water, which then boils to form steam. Temperature is recorded over time.
In the graph below the lower horizontal section represents melting(latent heat of fusion) and the higher horizontal section represents boiling(latent heat of vaporisation).
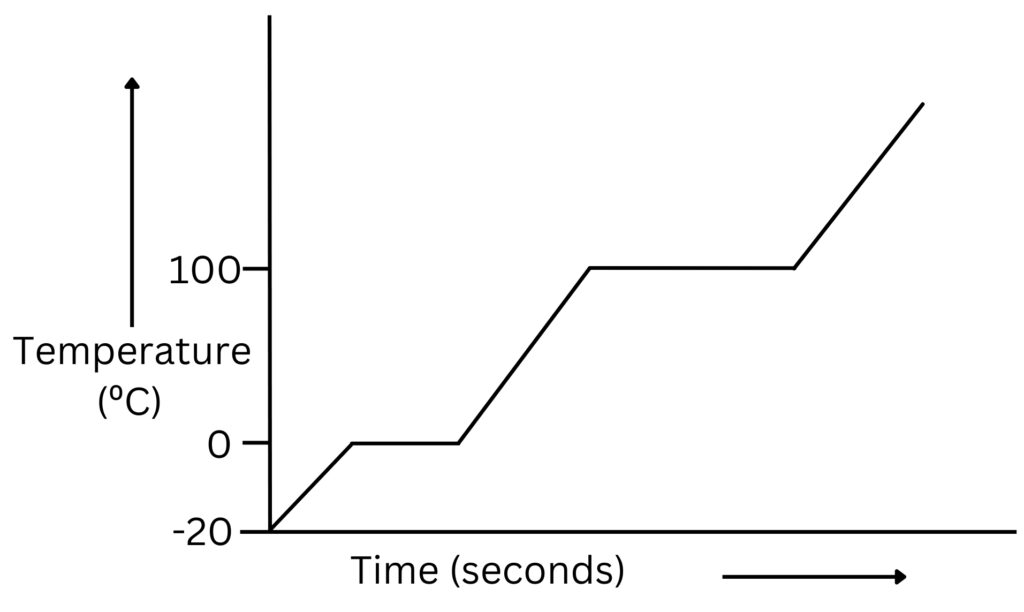
During both melting and boiling heat energy is still being supplied, but the temperature does not increase. This is because the energy being supplied increases the internal energy of the water.
Why does the internal energy increase when the substance is heated?
Between the particles there are tiny intermolecular forces that hold the particles together. These are stronger in solids, than in liquids.
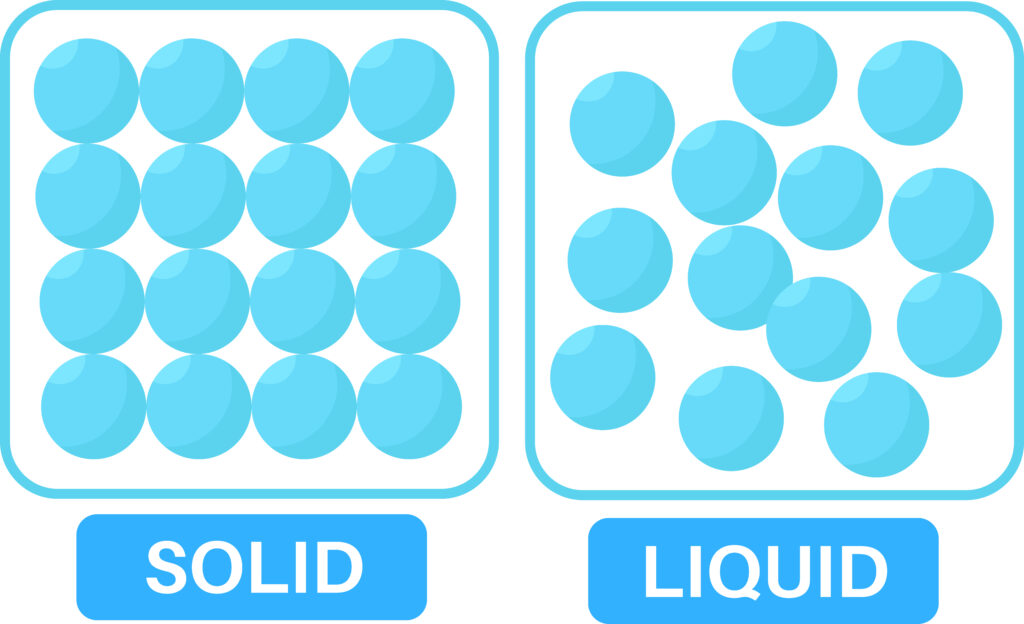
When the solid is heated, the internal energy of solid particles increases, so they vibrate faster. This causes the weak intermolecular forces to break as the solid melts to form a liquid
When the liquid is heated the internal energy of liquid particles increases, so they move faster, breaking the weak intermolecular forces between particles as the liquid evaporates to form a gas.
Practice Questions
1.State the definition of latent heat
2.Use the graph below to anwer the following question.

Suggest why the horizontal line for latent heat of vaporisation is longer than the horizontal line for latent heat of fusion
3. Explain how both the intermolecular forces and internal energy of water particles change as steam condenses to form liquid water
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations