GCSE JJ Thomson and Plum pudding model
J.J. Thomson

J.J. Thomson was a famous scientist who discovered the electron around 1897. After he discovered the electron, he proposed the plum pudding model of the atom.
The plum pudding model suggested that the atom is a ball of positive charge with negative electrons embedded in it.
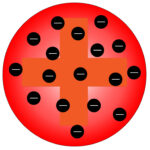
Rutherford carried out experiments which proved that the plum pudding model was incorrect, so it was later replaced by a new model.
This idea of starting with one model and replacing the model with a new model when new evidence appears is the way that Science works.
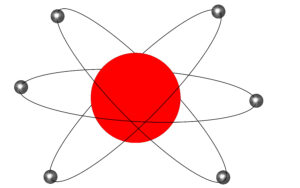
The model that replaced the plum pudding model is known as the nuclear model and is seen below:
Practice Questions
1. What famous particle did JJ Thomson discover
2. State the name of the atomic model that he proposed.
3. Describe the appearance of Thomson’s model
4. Why was Thomson’s model later rejected?
5. Which model replaced Thomson’s model?
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations