AQA GCSE Internal Energy
Internal Energy
A system is an object or group of objects. An object is made up of particles such as atoms or molecules. In the picture it shows a hot drink with water molecules forming steam above the cup.
Internal energy is the total energy stored inside the system by the particles.
This total internal energy is made up of the sum of both the total kinetic energy and the total potential energy of the particles.
Total Kinetic energy of the particles
The kinetic energy of the particles is due to their motion, relative to each other.
If the temperature of the system increases, then the kinetic energy of the particles will increase.
This is because for liquids or gases the particles move faster in random directions, in the case of solids the particles vibrate faster in a random direction.
As a result, when temperature increases, internal energy increases, because internal energy partly depends on kinetic energy.
Total Potential Energy
Potential energy is due to the position of the particles relative to each other.
In an experiment, ice is heated to form liquid water, heating is then continued to allow steam to form. The heat energy is supplied continuously as shown below
The graph below shows the changes in temperature over time.
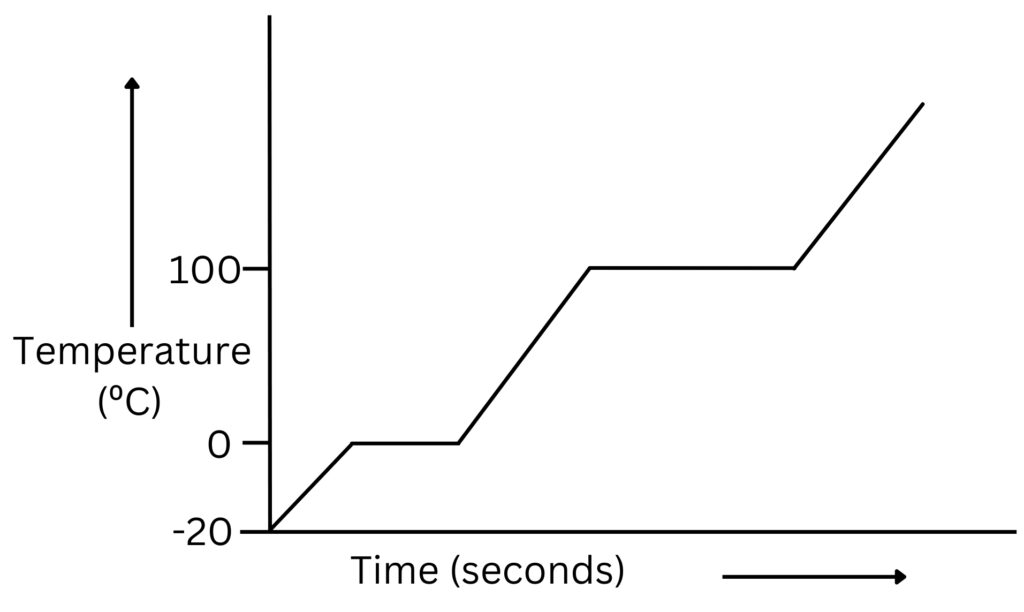
When the ice is melting at 0ºC and the water is boiling at 100ºC, although heat energy is being supplied, the temperature does not increase.
This is because the energy being supplied is being used to break forces of attraction between molecules (energy used is known as latent heat).
The energy supplied from heating the substance increases the potential store, rather than the kinetic store.
So, during a change of state, melting or boiling, there is a change in the potential energy.
Practice Questions
1.Define the term internal energy
2. Explain why internal energy increases when water is heated from 20ºC to 50ºC
3. Explain why internal energy increases when a solid is melting.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations