GCSE Different half lives of radioactive isotopes
Different half lives of radioactive isotopes
There are two possible definitions, either is suitable:
The half-life of a radioactive isotope is the time it takes for the number of nuclei of the isotope in a sample to halve.
The time it takes for the count rate (or activity) from a sample containing the isotope to fall to half its initial level.
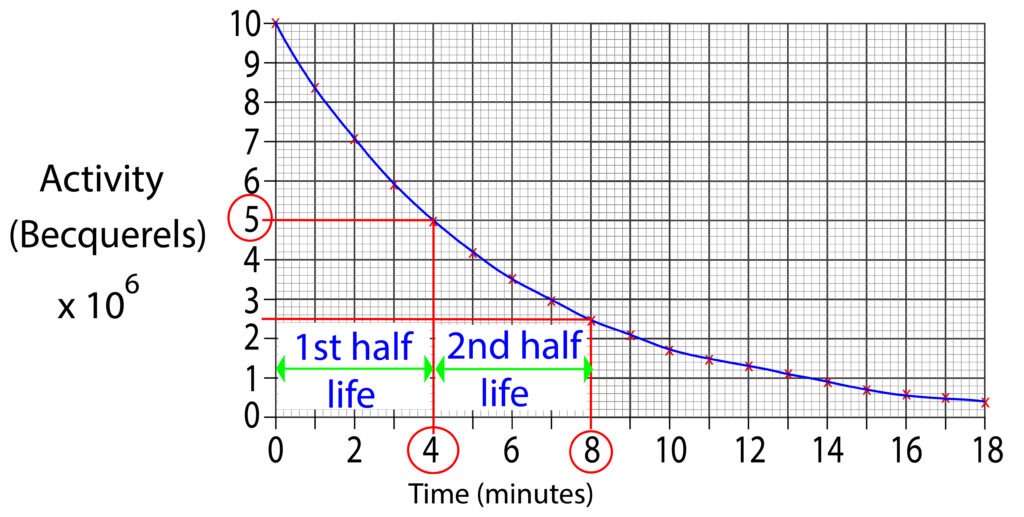
The half life for this radioactive isotope is 4 minutes. This is because every 4 minutes the activity will halve.
However, different radioactive isotopes have different half lives.
| Radioactive isotope | Half life |
|---|---|
| Iodine-131 | 8 days |
| Cobalt-60 | 5.3 years |
| Uranium-238 | 4.5 billion years |
Length of the half life
The length of the half life can indicate the hazard.
If the half life is very short, it can mean that a lot of radiation is emitted in a short time period, which can be hazardous. However, the material will decay quickly.
If the half life is very long, then the material takes a long time to decay, but it will emit the radiation over a longer time period.
When using radioactive sources, we need to consider the length of the half life, so it is long enough for our needs, but that it poses a minimum hazard to our health.
Practice Questions
1. Define the term half life
2. Which radioactive source Iodine-131 or Cobalt-60 is likely to emit more radiation over a 1 day time period?
3. Radioactive waste stays radioactive for tens of thousands of years, suggest why.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations