GCSE biofuels
Biofuels
A biofuel is a fuel has has been produced from either living organism, or one that was recently living.
We use this definition to prevent coal, oil and gas from being classed as biofuels. This is because coal, oil and gas were produced over millions of years.
Examples of biofuels include:
1.Sugar cane is harvested, fermented using yeast to make ethanol, distilled to purify and then burnt as a fuel.
2.Waste cooking oil is used to make biodiesel.
3. Logs, straw and coconut shells can all be burnt to release energy as a fuel
4. Biogas can be made using animal waste, see below.
Biogas Generators
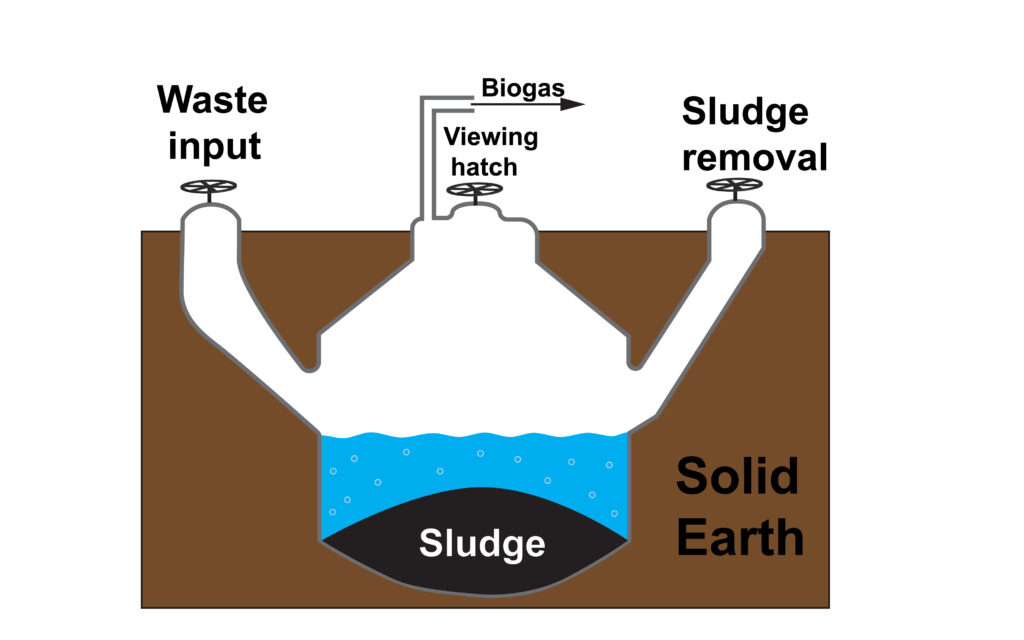
Biogas generators contain tank that is sunk into the ground. The ground acts as an insulator to help to maintain the temperature.
On the left the lid is opened, animal waste is added, then the lid is closed. Bacteria in the generator will break down the waste and produce biogas which is removed from the generator.
On the right, the lid is opened, a hose can be inserted to remove the sludge, which slowly builds up over time.
The biogas can be burnt as a fuel to release energy that can be used for:
1.Heating homes
2. Generating electricity
| Advantages of biofuels | Disadvantages of biofuels |
|---|---|
| Carbon neutral (see below) | Land space used for growing fuel crops cannot be used to grow food crops |
| Renewable | Growing crops takes a long time |
Carbon Neutral

Biofuels are carbon neutral, this means that the total amount of carbon dioxide produced when the fuel is burnt is equal to the total amount of carbon dioxide taken back in when the next batch of fuel crops are grown.
Practice Questions
1.Both coal and wood come from living organisms, but only wood is classed as a biofuel. Explain why wood is a biofuel, but coal is not.
2.Suggest why it is important to maintain the temperature in biogas generator.
3. What is the environmental advantage of using biogas to heat our homes compared to natural gas?
4.Explain why ethanol produced by fermentation is classed as carbon neutral.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations