Answers to GCSE Heating and Cooling Curves
Practice Questions
1. Write an experimental method of how to design an experiment to create a heating curve for ice forming steam
– Fill the beaker with a known quantity of ice cubes. Ensure the beaker is at room temperature.
– Place the thermometer or digital temperature probe in the beaker, making sure it is in contact with the ice but not touching the sides of the beaker.
– Record the initial temperature of the ice (this should be close to 0°C).
– Place the beaker on the electric hot plate or above the Bunsen burner. If using a Bunsen burner, adjust the flame to ensure a steady heat supply.
– Begin heating the ice gently. Start the stopwatch or timer as soon as heating begins.
– Record the temperature of the ice at regular intervals (e.g., every 30 seconds). Stir the ice-water mixture gently with the stirring rod to ensure uniform temperature distribution.
– Continue recording temperatures until the ice has completely melted, formed water which then boils to form steam.
– Plot the recorded temperatures against time to create a heating curve. The x-axis should represent time, and the y-axis should represent temperature.
– Identify and label the different phases on the graph: solid (ice), melting, liquid (water), boiling, and gas (steam).
2. Explain why part of the heating curve is horizontal
During a change of state, temperature will not change.
This is because if energy is being supplied to the substance for melting or boiling, internal energy of the particles increases, so weak intermolecular forces are being broken
If energy is being transferred away from the substance during condensing or freezing, internal energy of the particles decreases as weak intermolecular forces are being formed.
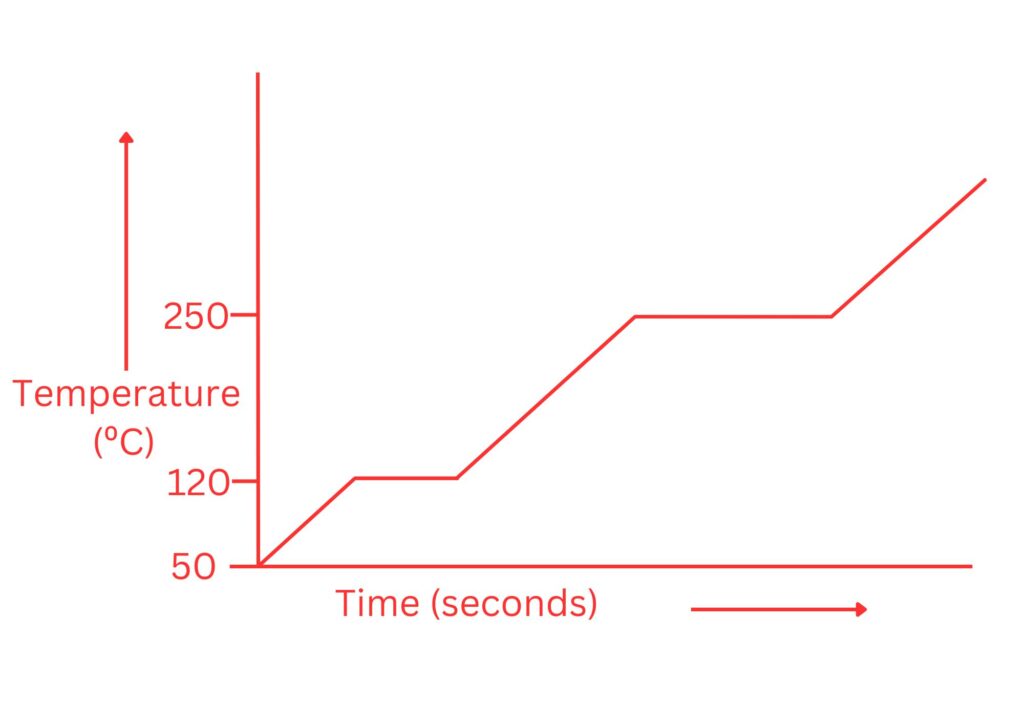
3. Benzoic acid has a melting point of 120ºC and a boiling point of 250ºC. Draw a heating curve to represent benzoic acid.
Absorption and Emission of EM Radiation
JJ Thomson and Plum pudding model
Ernest Rutherford and the Nuclear Model
Niels Bohr changing the Nuclear Model
Discovering the Proton and Neutron
Measuring radiation from radioactivity
Radiation types and properties
Random nature of radioactive decay
Radioactive contamination or irradiation
Hazards of contamination and irradiation
Studies on the effects of radiation on humans
Different half lives of radioactive isotopes
Nuclear Fission Chain Reaction
Writing nuclear fission equations