AQA GCSE Enzyme Rate Calculations(Biology)
Practice Questions
1. Catalase reacting with hydrogen peroxide will produce 60cm3 of oxygen gas in 90 seconds. Calculate the rate of reaction in cm3/s
Rate of reaction = 60/90 = 0.67cm3/s
2.Use the graph below to answer the following question

2a. Draw a tangent to the line, calculate the rate at 5 seconds
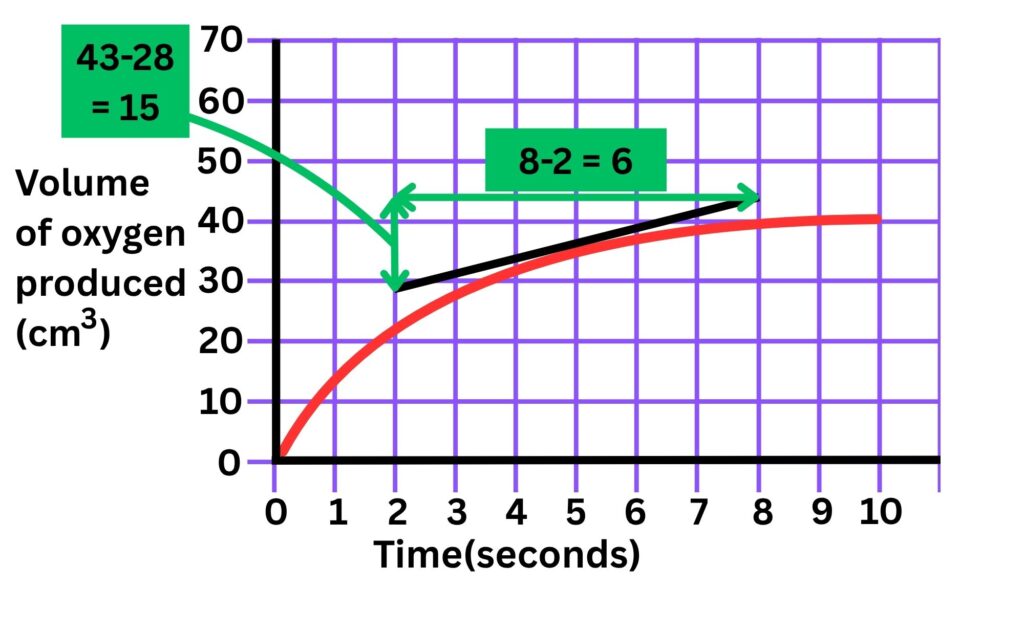
Rate of reaction = 15/6 = 2.5 cm3/s
2b. Describe how the rate changes as time increases
As time increases rate of reaction will decrease because the gradient is decreasing.
Factors affecting the rate of photosynthesis
Measuring & calculating rates of photosynthesis
Inverse square law and photosynthesis
Economics of enhancing the conditions in greenhouses
Investigating the effect of light intensity on the rate of photosynthesis